CURRENT ISSUE
September, 2025
No. 110 (9)
2024 Impact Factor: 7.9
2024 Journal Citation Indicator: 1.9
2024 CiteScore: 11.3
2024 Journal Citation Indicator: 1.9
2024 CiteScore: 11.3
EDITOR'S PICKS
Review Series on pediatric acute leukemias and myelodysplastic syndromes
Pediatric acute leukemias and myelodysplastic syndromes, progress and challenges: introduction to a review series
Acute therapy-related toxicities in pediatric acute lymphoblastic leukemia
Relapsed childhood T-cell acute lymphoblastic leukemia and lymphoblastic lymphoma
KMT2A-rearranged acute lymphoblastic leukemia in infants: current progress and challenges
Novel classification system and high-risk categories of pediatric acute myeloid leukemia
Germline and somatic genetic landscape of pediatric myelodysplastic syndromes
ARTICLES IN THREE SENTENCES
Article
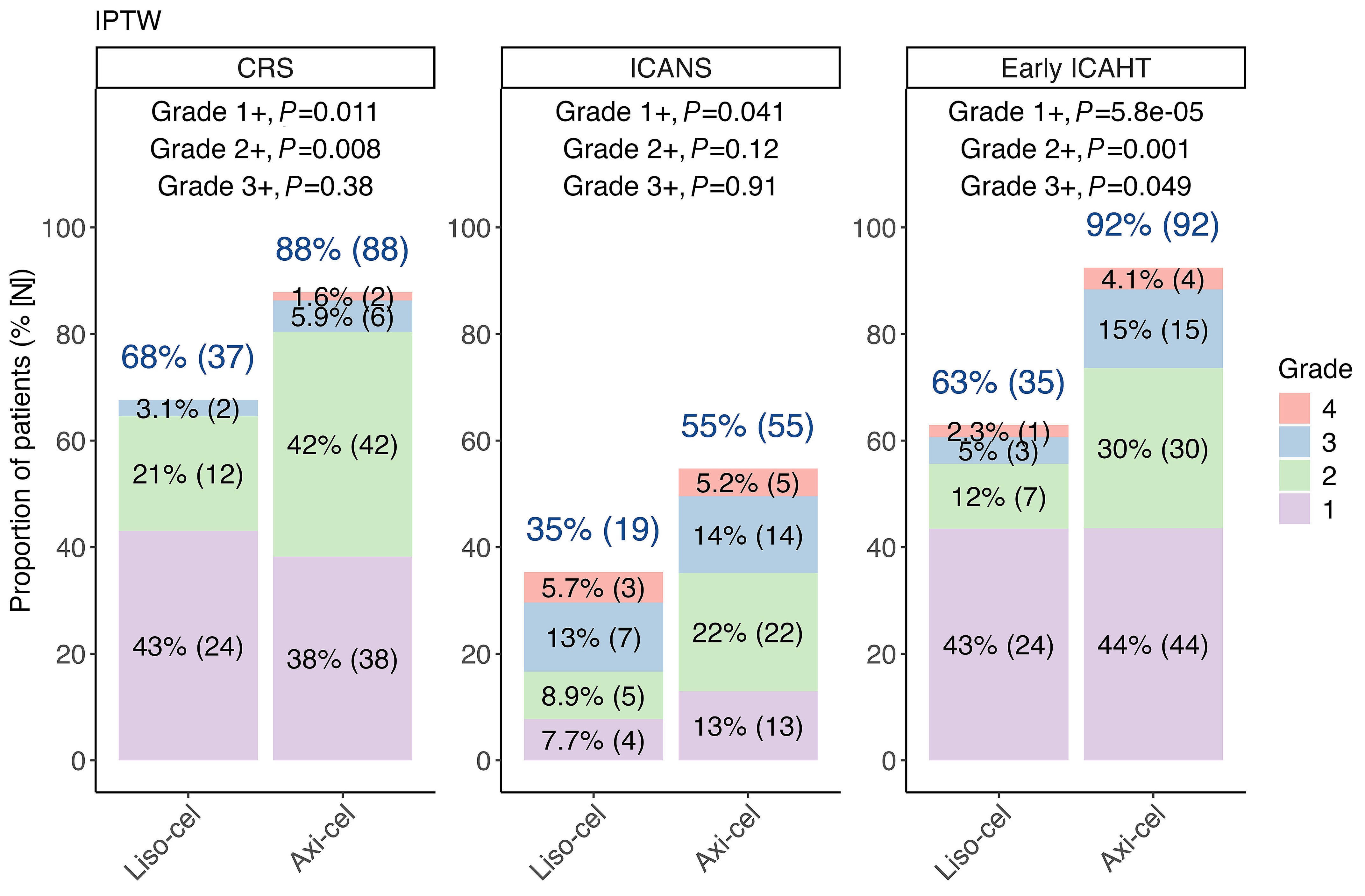
Real-world comparison of lisocabtagene maraleucel and axicabtagene ciloleucel in large B-cell lymphoma: an inverse probability of treatment weighting analysis with 3-year follow-up
Comparative real-world analyses of efficacy and toxicity of lisocabtagene maraleucel versus axicabtagene ciloleucel (axi-cel) in relapsed/refractory large B-cell lymphoma are lacking. Portuguese and colleagues provide a new real-world study using inverse probability of treatment weighting. Results show that although axi-cel is associated with greater toxicity requiring more intensive management, the response rates and survival outcomes of patients treated with these two CAR T-cell therapies are comparable.
Letter
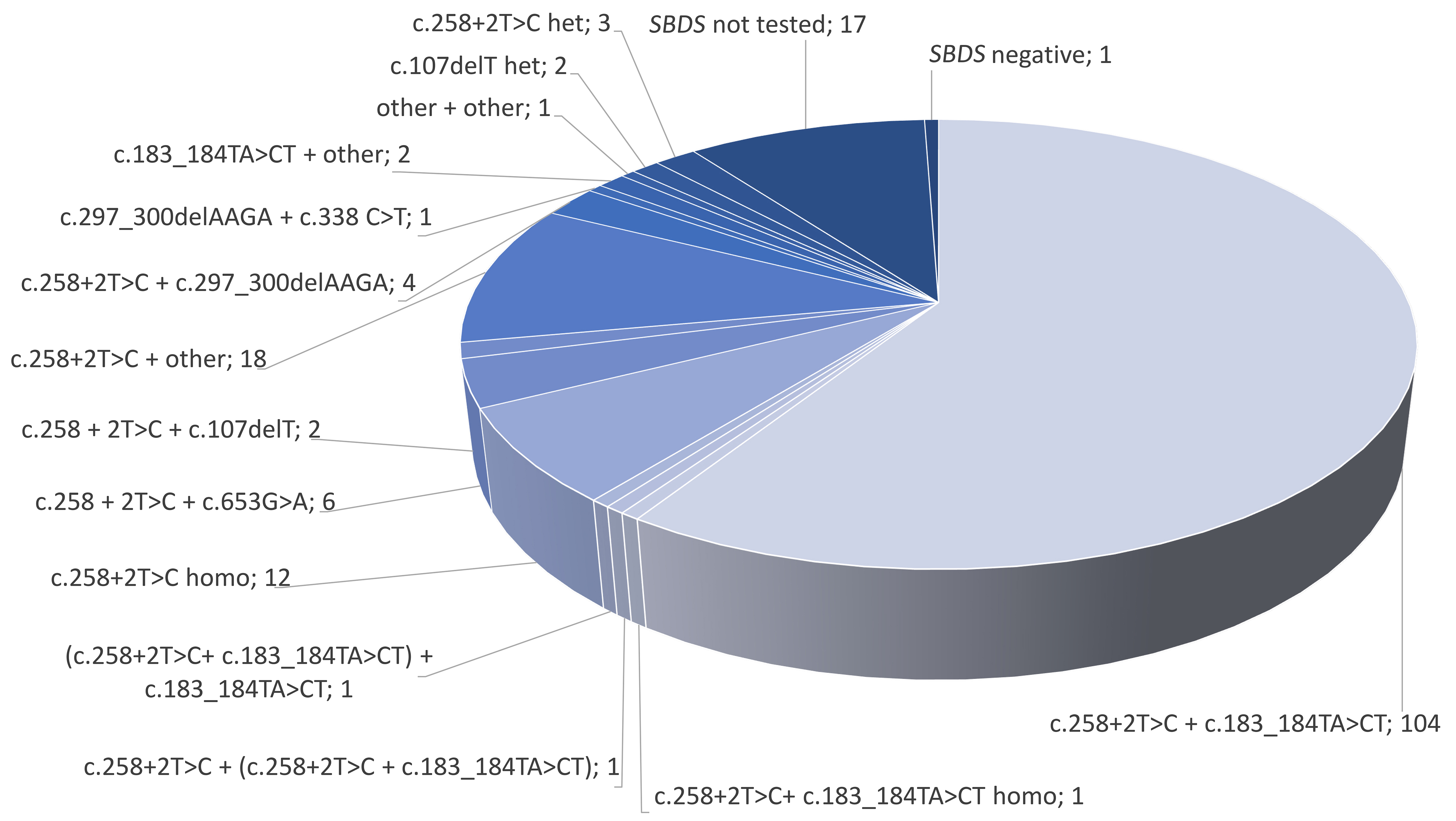
Genetic and clinical characteristics of patients with Shwachman Diamond syndrome with special consideration of treatment with granulocyte-colony stimulating factor
Shwachman-Diamond syndrome (SDS) is characterized, among other elements, by chronic neutropenia and an increased risk of bone marrow failure or leukemic transformation. Mellor-Heineke and colleagues evaluate the genomic landscape of SDS, clinical manifestations, and the effects of the controversial treatment with granulocyte-colony stimulating factor (G-CSF). Despite genetic homogeneity, they emphasize the variability in presentation and demonstrate that G-CSF therapy does not increase the risk of developing myelodysplastic syndrome or leukemia.
Article
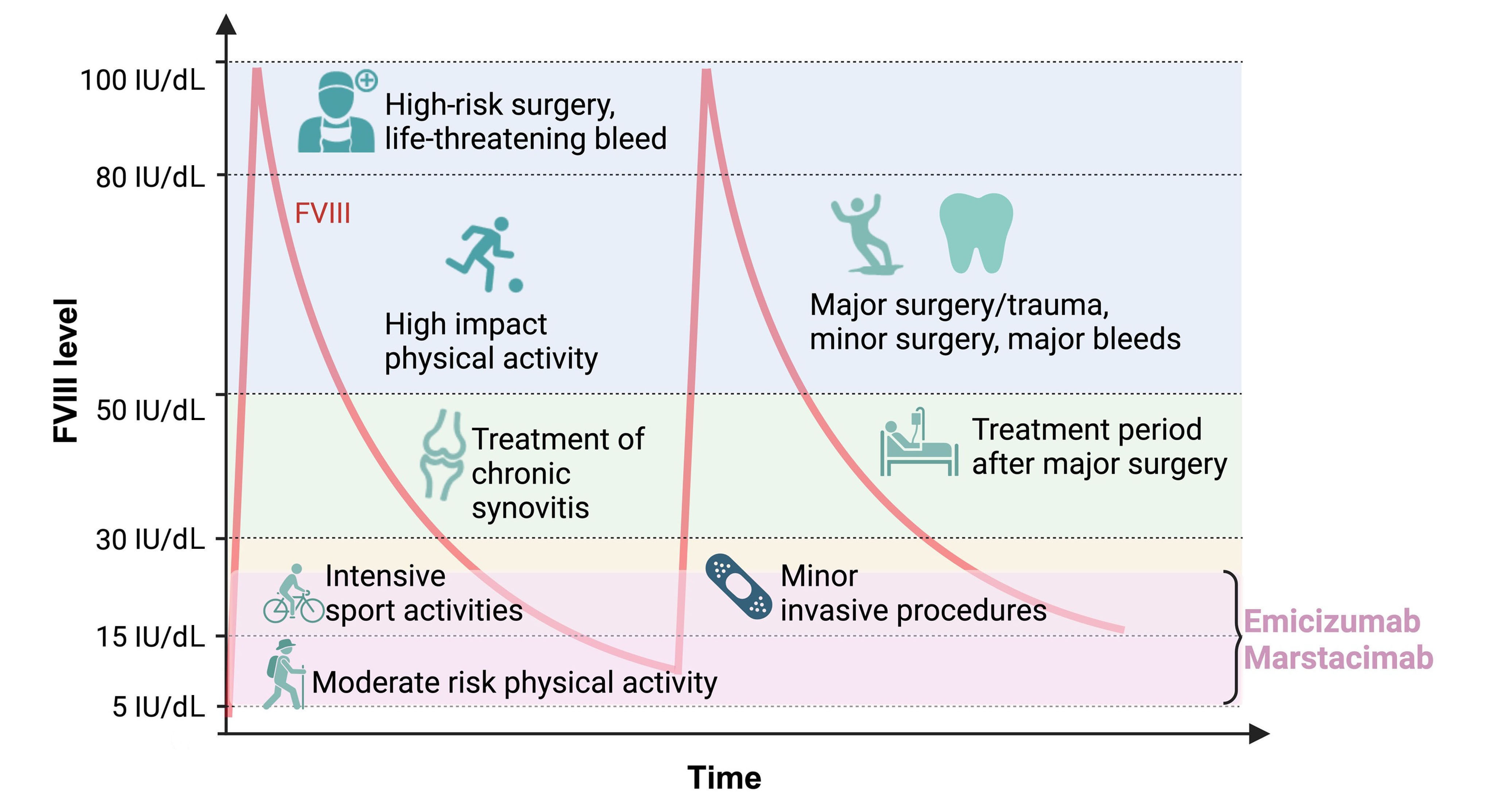
Consistent clinical factor VIII equivalency is unlikely for non-factor therapies in hemophilic mice
Non-factor therapies have changed the treatment paradigm of hemophilia A; however, it is not yet understood whether they are comparable to factor VIII (FVIII) in terms of mode of action and activity. Sefiane and colleagues performed in vivo testing of the non-factor molecules emicizumab and a sequence-identical analog of marstacimab (SIA-marstacimab) to evaluate whether or not there an absolute FVIII equivalence exists for these agents. Their data suggest that there is unlikely to be a single FVIII equivalence for emicizumab and SIA-marstacimab because their activity is dependent on local conditions and severity of the injury.
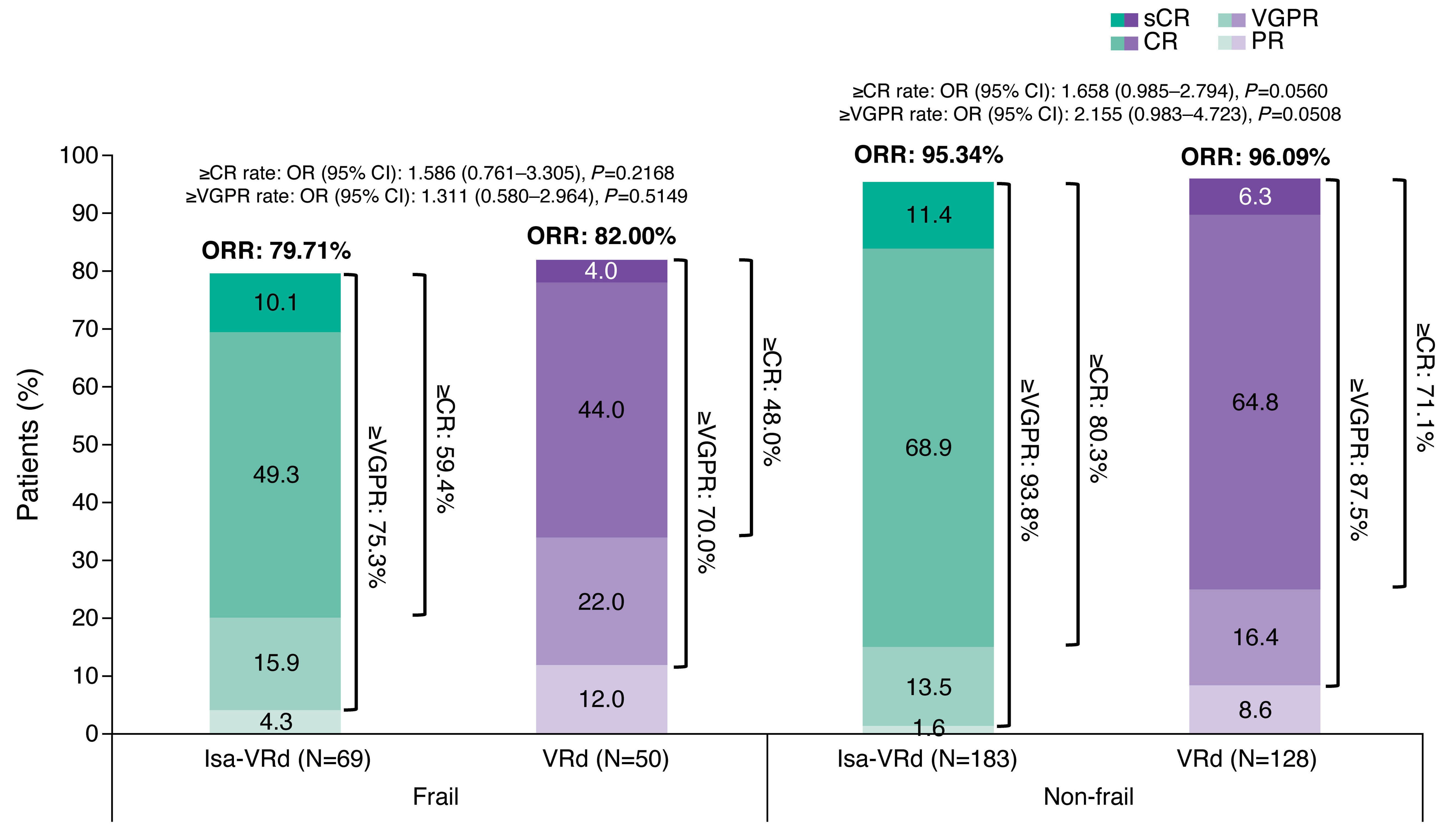
Article
Isatuximab plus bortezomib, lenalidomide, and dexamethasone for transplant-ineligible newly diagnosed multiple myeloma patients: a frailty subgroup analysis of the IMROZ trial
The anti-CD38 monoclonal antibody isatuximab (Isa) is approved in combination with bortezomib, lenalidomide, and dexamethasone (VRd) for newly diagnosed transplant-ineligible patients with multiple myeloma on the basis of the IMROZ study, a global, phase III, open-label study. Manier and colleagues present a post-hoc analysis of the IMROZ study across frail and non-frail subgroups using the simplified International Myeloma Working Group frailty score. Their results demonstrate that Isa-VRd quadruplet regimen is safe and efficacious in all patients aged ≤80 years not eligible for a transplant regardless of frailty status.
TAKE ADVANTAGE FROM HAEMATOLOGICA